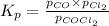Cocl2(g) decomposes according to the equation above. when pure cocl2(g) is injected into a rigid, previously evacuated flask at 690 k, the pressure in the flask is initially 1.0 atm. after the reaction reaches equilibrium at 690 k, the total pressure in the flask is 1.2 atm. what is the value of kp for the reaction at 690 k?

Answers: 3


Other questions on the subject: Chemistry



You know the right answer?
Cocl2(g) decomposes according to the equation above. when pure cocl2(g) is injected into a rigid, pr...
Questions in other subjects:










 for the reaction at 690 K is 0.05
for the reaction at 690 K is 0.05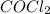 = 1.0 atm
= 1.0 atm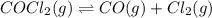
![[(1 - x) + x+ x]=1.2\\\\x=0.2atm](/tpl/images/0292/9648/696d1.png)