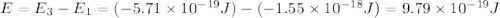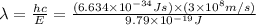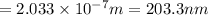Chemistry, 06.10.2019 05:30 kaylarae1930
Consider the following energy levels of a hypothetical atom: e4 −1.21 × 10−19 j e3 −5.71 × 10−19 j e2 −1.05 × 10−18 j e1 −1.55 × 10−18 j (a) what is the wavelength of the photon needed to excite an electron from e1 to e4? (b) what is the energy (in joules) a photon must have in order to excite an electron from e2 to e3? (c) when an electron drops from the e3 level to the e1 level, the atom is said to undergo emission. calculate the wavelength of the photon emitted in this process.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 15:30, 20cschultz
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 16:30, danbelucio
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
You know the right answer?
Consider the following energy levels of a hypothetical atom: e4 −1.21 × 10−19 j e3 −5.71 × 10−19 j e...
Questions in other subjects:

Physics, 15.02.2021 15:40

History, 15.02.2021 15:40


History, 15.02.2021 15:40



Chemistry, 15.02.2021 15:50

Mathematics, 15.02.2021 15:50

Mathematics, 15.02.2021 15:50

Business, 15.02.2021 15:50

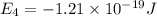
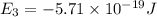
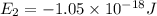
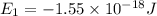
 to
to 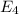 .
.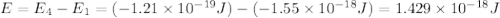
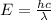
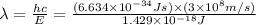
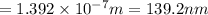
 to
to 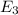 .
.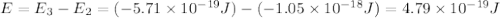
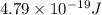 is the energy a photon to excite an electron from
is the energy a photon to excite an electron from