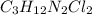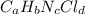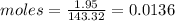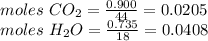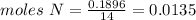Chemistry, 04.10.2019 20:20 estrellagutierrez12
Compound x contains only carbon, hydrogen, nitrogen, and chlorine. when 1.00 g of x is dissolved in water and allowed to react with excess silver nitrate, agno3, all the chlorine in x reacts and 1.95 g of solid agcl is formed. when 1.00 g of x undergoes complete combustion, 0.900 g of co2 and 0.735 g of h2o are formed. what is the empirical formula of x?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, aeverettpdzrvo
The most efficient way to establish the best possible economizer position is to measure
Answers: 1

Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2


Chemistry, 22.06.2019 20:00, teacherpreacher
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
You know the right answer?
Compound x contains only carbon, hydrogen, nitrogen, and chlorine. when 1.00 g of x is dissolved in...
Questions in other subjects:

Mathematics, 23.04.2020 17:24




Mathematics, 23.04.2020 17:24



Mathematics, 23.04.2020 17:24


Mathematics, 23.04.2020 17:25