
Astrip of magnesium metal having a mass of 1.30 g dissolves in 150. ml of 5.00 m hcl (specific gravity = 1.10); products of the reaction are magnesium chloride and hydrogen gas. the hcl is initially at 22.0°c, and the resulting solution reaches a final temperature of 52.0°c. the heat capacity of the calorimeter in which the reaction occurs is 345 j/°c. calculate δh (in kj/mol) for the reaction under the conditions of the experiment, assuming the specific heat of the final solution is the same as that for water (4.184 j/g°c).

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 11:00, jodonw5616
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1
You know the right answer?
Astrip of magnesium metal having a mass of 1.30 g dissolves in 150. ml of 5.00 m hcl (specific gravi...
Questions in other subjects:

Mathematics, 23.09.2019 17:30

History, 23.09.2019 17:30

English, 23.09.2019 17:30

Mathematics, 23.09.2019 17:30

Computers and Technology, 23.09.2019 17:30

Health, 23.09.2019 17:30

History, 23.09.2019 17:30



History, 23.09.2019 17:30

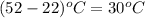
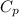 = 345
= 345 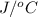
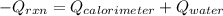
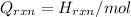
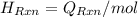
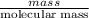
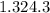
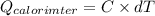
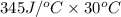
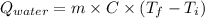
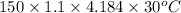
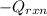 = (20710.8 + 10350) J
= (20710.8 + 10350) J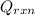 = -31060.8 J
= -31060.8 J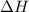 of the reaction as follows.
of the reaction as follows. 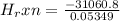
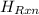 = -580684.24 J/mol
= -580684.24 J/mol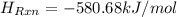 (as 1 kJ = 1000 J)
(as 1 kJ = 1000 J)

