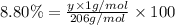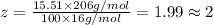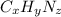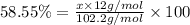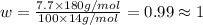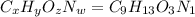Chemistry, 06.10.2019 02:30 xwalker6772
Ibuprofen, a headache remedy, contains 75.69% c, 8.80% h, and 15.51% o by mass and has a molar mass of 206 g/mol. express your answers as chemical formulas separated by a comma. nothing request answer part b cadaverine, a foul-smelling substance produced by the action of bacteria on meat, contains 58.55% c, 13.81% h, and 27.40% n by mass; its molar mass is 102.2 g/mol. express your answers as chemical formulas separated by a comma. nothing request answer part c epinephrine (adrenaline), a hormone secreted into the bloodstream in times of danger or stress, contains 59.0% c, 7.1% h, 26.2% o, and 7.7% n by mass; its molar mass is about 180 amu. express your answers as chemical formulas separated by a comma. nothing

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:40, alleshia2007
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 23:50, josie311251
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 07:30, klocke2001
Can you guys answer these questions i need it before 1: 00pm
Answers: 3

Chemistry, 23.06.2019 07:40, Aaron5795
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1
You know the right answer?
Ibuprofen, a headache remedy, contains 75.69% c, 8.80% h, and 15.51% o by mass and has a molar mass...
Questions in other subjects:

Biology, 03.02.2020 08:54


Mathematics, 03.02.2020 08:54


Chemistry, 03.02.2020 08:54


Mathematics, 03.02.2020 08:54



Health, 03.02.2020 08:54