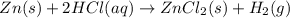When kay added 30 g of hydrochloric acid (hcl) to 65 g of zn, bubbles appeared and a white precipitate, zinc chloride (zncl2) was produced. she found that the mass of the zinc chloride was 93 g. since the law of conservation of matter stated that matter can't be created or destroyed, she was puzzled about the difference in mass. what is the best explanation for the missing 2 g?
a) 2 g of zinc disappeared.
b) 2 g of hydrogen gas were produced.
c) kay didn't correctly mass the precipitate.
d) kay didn't collect all of the precipitate.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:30, Karinaccccc
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 22.06.2019 22:50, kanerobertrosss2213
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3


Chemistry, 23.06.2019 01:30, mindofnyny
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1
You know the right answer?
When kay added 30 g of hydrochloric acid (hcl) to 65 g of zn, bubbles appeared and a white precipita...
Questions in other subjects:



Biology, 26.09.2019 21:20







Mathematics, 26.09.2019 21:20