
Three isotopes of argon occur in nature – 36 18ar, 38 18ar, 40 18ar. calculate the average atomic mass of argon to two decimal places, given the following relative atomic masses and the abundances of each of the isotopes: argon36 (35.97 amu; 0.337%), argon-38 (37.96 amu; 0.063%), argon-40 (39.96 amu; 99.600%).

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:00, QuestionsAnsweredNow
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3


Chemistry, 23.06.2019 01:30, rubyr9975
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1
You know the right answer?
Three isotopes of argon occur in nature – 36 18ar, 38 18ar, 40 18ar. calculate the average atomic ma...
Questions in other subjects:


Spanish, 01.07.2019 13:00


Mathematics, 01.07.2019 13:00

English, 01.07.2019 13:00




Health, 01.07.2019 13:00

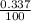 x 35.97) + (
x 35.97) + ( x 37.96) +(
x 37.96) +(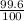 x 39.96)
x 39.96)


