
Chemistry, 02.10.2019 21:30 Milanchik28
When 21.7 ml of 0.500 m h2so4 is added to 21.7 ml of 1.00 m koh in a coffee-cup calorimeter your question has been answered let us know if you got a answer. rate this answer question: when 21.7 ml of 0.500 m h2so4 is added to 21.7 ml of 1.00 m koh in a coffee-cup calorimeter at when 21.7 ml of 0.500 m h2so4 is added to 21.7 ml of 1.00 m koh in a coffee-cup calorimeter at 23.50°c, the temperature rises to 30.17°c. calculate δh of this reaction. (assume that the total volume is the sum of the individual volumes and that the density and specific heat capacity of the solution are the same as for pure water.) (d for water = 1.00 g/ml; c for water = 4.184 j/g·°c.) kj/mol h2o ?

Answers: 3


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 18:00, heggestade
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
When 21.7 ml of 0.500 m h2so4 is added to 21.7 ml of 1.00 m koh in a coffee-cup calorimeter your qu...
Questions in other subjects:

Social Studies, 02.05.2021 02:30



Mathematics, 02.05.2021 02:30



Health, 02.05.2021 02:30

Business, 02.05.2021 02:30


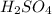 = 0.500 M
= 0.500 M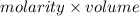
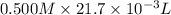
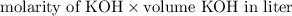
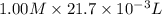
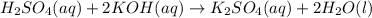
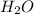 formed =
formed = 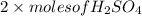 = (moles of KOH)
= (moles of KOH)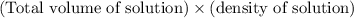
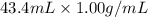
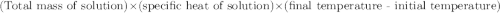
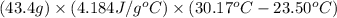
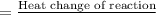
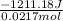
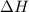 of the given reaction is -55.8 kJ/mol.
of the given reaction is -55.8 kJ/mol.

