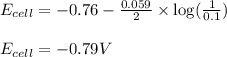Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, isaiahrodriguezsm17
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1



Chemistry, 23.06.2019 12:30, bryantjorell
An atom holds 7 electrons. use orbital notation to model the probable location of its electrons. an atom hold 22 electrons. use orbital notation to model the probable location of its electrons. an atom holds 17 electrons. use orbital notation to model the probable location of its electrons.
Answers: 1
You know the right answer?
Azinc rod is placed in 0.1 mznso4 solution at 298 k. write the electrode reaction and calculate the...
Questions in other subjects:


Mathematics, 22.07.2020 05:01








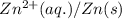

![E_{(Zn^{2+}/Zn)}=E^o_{(Zn^{2+}/Zn)}-\frac{0.059}{n}\log \frac{[Zn]}{[Zn^{2+}]}](/tpl/images/0284/2267/10ca8.png)
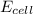 = electrode potential of the cell = ?V
= electrode potential of the cell = ?V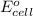 = standard electrode potential of the cell = -0.76 V
= standard electrode potential of the cell = -0.76 V![[Zn]=1M](/tpl/images/0284/2267/0b1ce.png) (concentration of pure solids are taken as 1)
(concentration of pure solids are taken as 1)![[Zn^{2+}]=0.1M](/tpl/images/0284/2267/ed5b8.png)