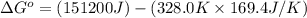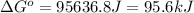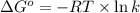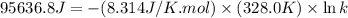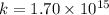For the reaction fe3o4(s) + 4h2(g) --> 3fe(s) + 4h2o(g)
h° = 151.2 kj and s° = 169.4...

Chemistry, 02.10.2019 21:30 bluebabyyy
For the reaction fe3o4(s) + 4h2(g) --> 3fe(s) + 4h2o(g)
h° = 151.2 kj and s° = 169.4 j/k
the equilibrium constant for this reaction at 328.0 k is

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 11:40, tatemelliott
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3
You know the right answer?
Questions in other subjects:


Mathematics, 29.06.2019 21:00


Mathematics, 29.06.2019 21:00



English, 29.06.2019 21:00


Mathematics, 29.06.2019 21:00


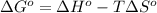
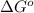 = standard Gibbs free energy = ?
= standard Gibbs free energy = ?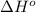 = standard enthalpy = 151.2 kJ = 151200 J
= standard enthalpy = 151.2 kJ = 151200 J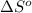 = standard entropy = 169.4 J/K
= standard entropy = 169.4 J/K