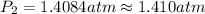Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:50, ladybugperez05
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1
You know the right answer?
The vapor pressure of water is 1.00 atm at 373 k, and the enthalpy of vaporization is 40.68 kj mol!...
Questions in other subjects:


Chemistry, 29.10.2020 09:00


History, 29.10.2020 09:00


Mathematics, 29.10.2020 09:00

English, 29.10.2020 09:00

Mathematics, 29.10.2020 09:00


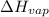 of the reaction, we use clausius claypron equation, which is:
of the reaction, we use clausius claypron equation, which is:![\ln(\frac{P_2}{P_1})=\frac{\Delta H_{vap}}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0284/2756/b9a49.png)
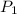 = vapor pressure at temperature
= vapor pressure at temperature 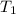
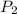 = vapor pressure at temperature
= vapor pressure at temperature 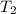
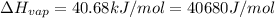
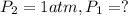
![\ln(\frac{1 atm}{P_1})=\frac{40680 J/mol}{8.314J/mol.K}[\frac{1}{363}-\frac{1}{373}]](/tpl/images/0284/2756/cf798.png)
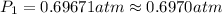
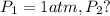
![\ln(\frac{P_2}{1 atm})=\frac{40680 J/mol}{8.314J/mol.K}[\frac{1}{373}-\frac{1}{383}]](/tpl/images/0284/2756/01f21.png)