
Butylated hydroxytoluene (bht) is used as an antioxidant in processed foods. (it prevents fats and oils from becoming rancid.) a solution of 2.500 g of bht in 100.0 g of benzene had a freezing point of 4.880 oc. what is molecular mass of bht? . kf for benzene is 5.065 oc/m

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, thebrain1345
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2


Chemistry, 22.06.2019 21:50, namoralessimon03
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1
You know the right answer?
Butylated hydroxytoluene (bht) is used as an antioxidant in processed foods. (it prevents fats and o...
Questions in other subjects:


Social Studies, 09.04.2020 17:29



Mathematics, 09.04.2020 17:29


English, 09.04.2020 17:29



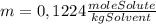
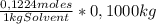 = 0,01224 moles of solute ≡ BHT
= 0,01224 moles of solute ≡ BHT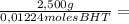 204,2 g/mol
204,2 g/mol

