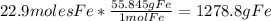Chemistry, 02.10.2019 21:20 fancycar14
Iron(iii) oxide, fe2o3, is a result of the reaction of iron with the oxygen in air. a. what is the balanced equation for this reaction? (use the lowest possible coefficients. omit states of matter.) b. what number of moles of iron reacts with 17.15 mol of oxygen from the air? mol c. what mass of iron is required to react with 17.15 mol of oxygen? mass =

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, pollywallythecat
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 17:40, aaliyahthomas37
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

You know the right answer?
Iron(iii) oxide, fe2o3, is a result of the reaction of iron with the oxygen in air. a. what is the b...
Questions in other subjects:










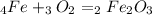
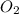
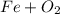
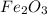 , so you should write the product:
, so you should write the product: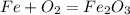
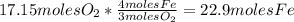
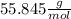 , so:
, so: