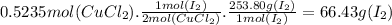Chemistry, 02.10.2019 21:10 blachaze8729
12 is produced by the reaction of 0.5235 mol of cucl2 according to the following equation: 2cucl2 +4k1—2cul + 4kci + 12. what is the mass of the la produced? 66.43 g 33.22 g 132.9 g

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, sdlesley66
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 10:00, halohero7
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 23.06.2019 00:00, familyk0jj3
How do you determine the percent yield of a chemical reaction
Answers: 1

Chemistry, 23.06.2019 00:30, danielmartinez024m
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1
You know the right answer?
12 is produced by the reaction of 0.5235 mol of cucl2 according to the following equation: 2cucl2 +...
Questions in other subjects:

English, 30.03.2021 14:00


Biology, 30.03.2021 14:00

English, 30.03.2021 14:00

English, 30.03.2021 14:00

Social Studies, 30.03.2021 14:00

Biology, 30.03.2021 14:00

English, 30.03.2021 14:00

Chemistry, 30.03.2021 14:00