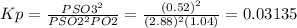Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 500 ml flask with 3.4 atm of sulfur dioxide gas and 1.3 atm of oxygen gas at 31. °c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of sulfur trioxide gas to be 0.52 atm. calculate the pressure equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answert significant digits. x i ? explanation

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:30, camerondillonn
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1


You know the right answer?
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid...
Questions in other subjects:


Arts, 07.04.2021 01:00

Mathematics, 07.04.2021 01:00



Mathematics, 07.04.2021 01:00


Health, 07.04.2021 01:00