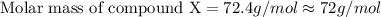When 5.42 g of a certain molecular compound x are dissolved in 80.0 g of formamide (nh, coh), the freezing point of the solution is measured to be -1.4 °c. calculate the molar mass of x. if you need any additional information on formamide, use only what you find in the aleks data resource. also, be sure your answer has a unit symbol, and is rounded to 2 significant digits. one x 6 ?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, coolkid2041
Calculate the number of moles of ethane in 100 grams
Answers: 3

Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 22.06.2019 17:30, kaytonleeb
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 22:50, kanerobertrosss2213
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
When 5.42 g of a certain molecular compound x are dissolved in 80.0 g of formamide (nh, coh), the fr...
Questions in other subjects:


English, 28.07.2019 18:30

Biology, 28.07.2019 18:30

History, 28.07.2019 18:30

English, 28.07.2019 18:30



Arts, 28.07.2019 18:30


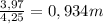
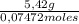 = 73 g/mol
= 73 g/mol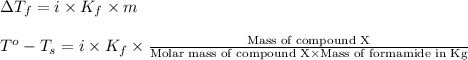
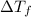 = change in freezing point
= change in freezing point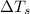 = freezing point of solution =
= freezing point of solution = 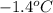
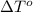 = freezing point of formamide =
= freezing point of formamide = 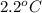
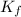 = freezing point constant for formamide =
= freezing point constant for formamide = 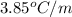
![[2.2-(-1.4)]^oC=1\times (3.85^oC/m)\times \frac{5.42g}{\text{Molar mass of compound X}\times 0.080kg}](/tpl/images/0284/0592/99dda.png)