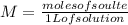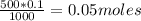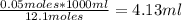Chemistry, 02.10.2019 21:00 martinezizzie
The molarity of "concentrated" hcl is approximately 12.1 m. how many milliliters of this reagent should be diluted to 500 ml to make 0.100 m hcl?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:40, taysomoneyyy
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2


Chemistry, 22.06.2019 06:30, Pizzapegasus1
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1
You know the right answer?
The molarity of "concentrated" hcl is approximately 12.1 m. how many milliliters of this reagent sho...
Questions in other subjects:


Mathematics, 16.07.2020 23:01


Social Studies, 16.07.2020 23:01


Biology, 17.07.2020 01:01

Mathematics, 17.07.2020 01:01

Mathematics, 17.07.2020 01:01

Mathematics, 17.07.2020 01:01