
Chemistry, 02.10.2019 21:00 sandeebassett3
Common brass is a copper and zinc alloy containing 37.0% zinc by mass and having a density of 8.48 g/cm3. a fitting composed of common brass has a total volume of 122.5 cm3 .
how many atoms of copper does the fitting contain?
how many atoms of zinc does the fitting contain?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:00, miguel454545
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 23:30, emmalado45
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 03:00, Cheyenne7327
Analyze the reaction to determine whether the reaction is exothermic or endothermic. explain your reasoning.
Answers: 1

Chemistry, 23.06.2019 07:50, dootdootkazoot
What is the significance sodium hydroxide and hydrochloric acid
Answers: 1
You know the right answer?
Common brass is a copper and zinc alloy containing 37.0% zinc by mass and having a density of 8.48 g...
Questions in other subjects:


Health, 14.04.2020 16:50








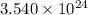
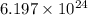
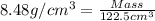
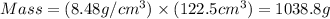
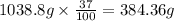
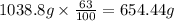
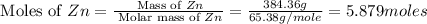
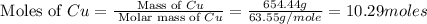
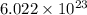 number of copper atoms
number of copper atoms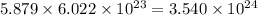 number of copper atoms
number of copper atoms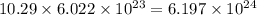 number of zinc atoms
number of zinc atoms

