
Chemistry, 02.10.2019 20:20 rebecca7415
A585−g piece of copper tubing is heated to 89.5°c and placed in an insulated vessel containing 159 g of water at 22.8°c. assuming no loss of water and a heat capacity for the vessel of 10.0 j/°c, what is the final temperature of the system (c of copper = 0.387 j/g·°c)?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, fgcherubin
Consider the balanced equation below. n2h4 + 2h2o2 n2 + 4h2o what are the mole ratios of hydrazine (n2h4) to hydrogen peroxide (h2o2) and hydrazine to water? 1: 2 and 1: 4 1: 3 and 1: 4 1: 2 and 3: 5 1: 3 and 3: 5
Answers: 3


You know the right answer?
A585−g piece of copper tubing is heated to 89.5°c and placed in an insulated vessel containing 159 g...
Questions in other subjects:


Mathematics, 10.11.2021 02:40

Mathematics, 10.11.2021 02:40


Business, 10.11.2021 02:40

Computers and Technology, 10.11.2021 02:40




History, 10.11.2021 02:40

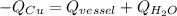 (1)
(1)

