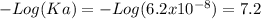Chemistry, 02.10.2019 20:20 keving4three
Aphosphate buffer is involved in the formation of urine. the developing urine contains h2po4 and hpo42- in the same concentration as present in blood plasma. identify the acid and its conjugate base. write the ionization equation and the ka expression. 6.2 x 10^(8) a. the ka of h2po4 is 6.2 x 108. what is the pka? is this acid weaker or stronger than h2co3? which buffer system is more optimal for regulating ph in the body? b. using lechatlier's principle, what happens if the urine is acidified (h+ ions added)?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, mpchop
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3


Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3
You know the right answer?
Aphosphate buffer is involved in the formation of urine. the developing urine contains h2po4 and hpo...
Questions in other subjects:


Mathematics, 09.11.2019 04:31

Mathematics, 09.11.2019 04:31

Mathematics, 09.11.2019 04:31



Mathematics, 09.11.2019 04:31


Social Studies, 09.11.2019 04:31

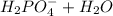 ⇄
⇄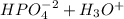 (1)
(1)![Ka = \frac{[HPO_{4}^{-2}] [H_{3}O^{+}]}{[H_{2}PO_{4}^{-}] [H_{2}O]} = 6.2x10^{-8}](/tpl/images/0284/0159/77441.png)