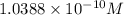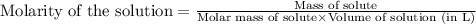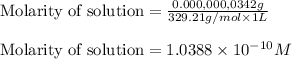Chemistry, 02.10.2019 20:10 mmagee2020
What is the molarity of .342 grams of adenosine 3'5' cyclic monophosphate (camp) in one lite

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, kaliyab191
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2



Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
You know the right answer?
What is the molarity of .342 grams of adenosine 3'5' cyclic monophosphate (camp) in one lite...
Questions in other subjects:

Mathematics, 17.09.2019 18:30


History, 17.09.2019 18:30

Mathematics, 17.09.2019 18:30


English, 17.09.2019 18:30




English, 17.09.2019 18:30