
Chemistry, 02.10.2019 20:10 21ghostrider21
Describe how you would prepare 100 ml of a 0.050 m sodium citrate tribasic buffer to a ph of 6. this method is accomplished by preparing a solution of a weak species base and then adding the appropriate strong species 0.5 m hcl to get to the desired ph. what amount of mass or volumes should you add?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:40, sadcase85
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na, so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1


Chemistry, 23.06.2019 03:00, jaidencoolman2866
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1
You know the right answer?
Describe how you would prepare 100 ml of a 0.050 m sodium citrate tribasic buffer to a ph of 6. this...
Questions in other subjects:


Mathematics, 21.11.2019 06:31








![\frac{[A^{-}]}{[HA]}](/tpl/images/0284/0047/5980f.png)
![\frac{[CitrateTribasic]}{[CitrateDibasic]}](/tpl/images/0284/0047/f66e1.png)
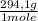 = 1,4705 g of sodium citrate tribasic dihydrate -commercial reactant-
= 1,4705 g of sodium citrate tribasic dihydrate -commercial reactant-


