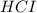Hydrocyanic acid, hcn, is a weak acid. (a) write the chemical equation for the dissociation of hcn in water. (b) identify the brønsted-lowry conjugate acid-base pairs in the equation above. (c) write the chemical equation for the reaction of hcn with naoh. (d) write the chemical equation for dissociation of nacn in water. (e) write the chemical equation for the reaction of nacn and hci.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1


Chemistry, 22.06.2019 23:40, sydneykated
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 00:30, Keemdadream13
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1
You know the right answer?
Hydrocyanic acid, hcn, is a weak acid. (a) write the chemical equation for the dissociation of hcn i...
Questions in other subjects:


Mathematics, 29.03.2021 23:40


Arts, 29.03.2021 23:40




Mathematics, 29.03.2021 23:40

History, 29.03.2021 23:40

Chemistry, 29.03.2021 23:40


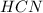 : acid
: acid 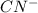 :conjugate base.
:conjugate base.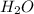 : base
: base 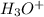 : conjugate acid.
: conjugate acid.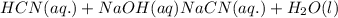
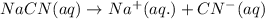
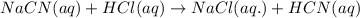
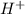 ions in their aqueous states.
ions in their aqueous states.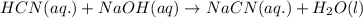
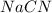 in water.
in water.