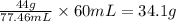Chemistry, 02.10.2019 20:00 elijahjacksonrp6z2o7
A44% wt/wt solution of h2so4 has a density of 1.343g/ml. what mass of h2so4 is 60ml of this solution?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, jaylanmahone223
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 14:00, leahstubbs
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 16:50, brandiwingard
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
A44% wt/wt solution of h2so4 has a density of 1.343g/ml. what mass of h2so4 is 60ml of this solution...
Questions in other subjects:


Mathematics, 21.07.2020 19:01

Mathematics, 21.07.2020 19:01


Biology, 21.07.2020 19:01

Mathematics, 21.07.2020 19:01

Social Studies, 21.07.2020 19:01

Mathematics, 21.07.2020 19:01


Mathematics, 21.07.2020 19:01