
Chemistry, 02.10.2019 03:00 bowmanari2154
Consider the nucleophilic addition reaction of 2‑butanone with excess propyl magnesiumbromide, made in situ by reacting 1‑bromopropane with metallic magnesium, to make 3‑methyl‑3‑hexanol. 2-butanone 1-bromopropane magnesium 3-methyl-3-hexanol =0.81 g/ml =1.35 g/ml =0.82 g/ml a reaction was performed in which 0.40 ml0.40 ml of 2‑butanone was reacted with an excess of propyl magnesiumbromide to make 0.38 g0.38 g of 3‑methyl‑3‑hexanol. calculate the theoretical yield and percent yield for this reaction.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, annarain2004
Asample of gas occupies 17 ml at –112°c. what volume does the sample occupy at 70°c a. 10.6 ml b. 27 ml c. 36 ml d. 8.0 ml you
Answers: 1

Chemistry, 22.06.2019 09:00, Aminton737
Plz mark brainliest 30 points1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s.2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 09:00, pinapunapula
Which explanation is true about what happens to a ray of light when it strikes a mirror? a. a light ray is transmitted toward a mirror at a certain angle. the light ray is then reflected by the mirror at an equal angle but in the opposite direction of the transmitted ray. b. an incident ray strikes a mirror at an angle with a line perpendicular to the mirror. the light ray is then reflected at an angle equal to the angle of incidence but on the opposite side of the perpendicular line. c. a reflecting ray strikes a mirror at an angle with a line perpendicular to the mirror. the light ray is then refracted at an angle equal to the angle of the reflecting ray and on the same side of the perpendicular line. d. an incident ray strikes a mirror at an angle with a line parallel to the mirror. the light ray is then transmitted at an angle equal to the angle of incidence but on the opposite side of the parallel line. you so much! : -d take the time to try and answer correctly.
Answers: 3

Chemistry, 22.06.2019 09:10, cheesedoodle
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1
You know the right answer?
Consider the nucleophilic addition reaction of 2‑butanone with excess propyl magnesiumbromide, made...
Questions in other subjects:





History, 02.12.2021 01:50


Biology, 02.12.2021 01:50

Mathematics, 02.12.2021 01:50


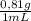 ×
×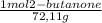 ×
×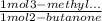 ×
×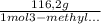 = 0,52 g
= 0,52 g

