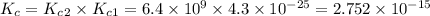Chemistry, 01.10.2019 23:00 morgaaaan651
The following reactions have the indicated equilibrium constants at a particular temperature: n2(g) + o2(g) ⇌ 2no(g)kc = 4.3 × 10−25 2no(g) + o2(g) ⇌ 2no2(g)kc = 6.4 × 109 determine the value of the equilibrium constant for the following equation at the same temperature: n2(g) + 2o2(g) ⇌ 2no2(g) kc = × 10 (enter your answer in scientific notation.)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, angelinararr5783
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1

Chemistry, 22.06.2019 09:30, jewelz5887
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 10:00, 2019reynolds
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 11:30, chelseychew32
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2
You know the right answer?
The following reactions have the indicated equilibrium constants at a particular temperature: n2(g)...
Questions in other subjects:

Social Studies, 30.04.2021 08:40

History, 30.04.2021 08:40

Mathematics, 30.04.2021 08:40

English, 30.04.2021 08:40


Health, 30.04.2021 08:40

Mathematics, 30.04.2021 08:40

Mathematics, 30.04.2021 08:40


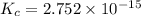
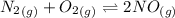
![K_c_1=\frac {[NO]^2}{[N_2][O_2]}=4.3\times 10^{-25}](/tpl/images/0281/1482/cff32.png)
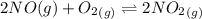
![K_c_2=\frac {[NO_2]^2}{[NO]^2[O_2]}=6.4\times 10^{9}](/tpl/images/0281/1482/9df1f.png)
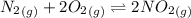
![K_c=\frac {[NO_2]^2}{[N_2][O_2]^2}](/tpl/images/0281/1482/1467b.png)
![[NO]^2](/tpl/images/0281/1482/b4f90.png) and rearranging in the above equation as:
and rearranging in the above equation as:![K_c=\frac {[NO_2]^2}{[NO]^2[O_2]}\times \frac {[NO]^2}{[N_2][O_2]}](/tpl/images/0281/1482/822cf.png)