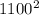Chemistry, 01.10.2019 21:00 tanyiawilliams14991
The diagram shows two samples of gas enclosed in identical containers. each colored ball represents a gas particle. both samples have the same number of particles.
sample a
mass of each particle: 28 u
average particle speed: 980 m/s
sample b
mass of each particle: 44 u
average particle speed: 1,100 m/s
compare the average kinetic energies of the particles in each sample. which sample has the
higher temperature?
sample a
sample b
neither, both samples are the same.
on

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:20, kingaman
Which of the following statements is not true? • a. covalent compounds have low melting and boiling points. • ob. covalent bonds between atoms of a compound are relatively weak compared to bonds between molecules. • c. covalent bonds occur between nonmetals. • d. covalent compounds are often gases or liquids.
Answers: 2

Chemistry, 22.06.2019 05:00, YoEsMyles3115
0.2348 grams of pbcl2 used to form 44.0 ml of solution.
Answers: 1


Chemistry, 22.06.2019 14:30, CoolRahim9090
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1
You know the right answer?
The diagram shows two samples of gas enclosed in identical containers. each colored ball represents...
Questions in other subjects:

Mathematics, 27.08.2020 14:01







Biology, 27.08.2020 14:01


Health, 27.08.2020 14:01

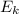 = 1/2 * m *
= 1/2 * m * 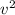
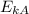 =
= 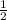 * 28 *
* 28 * 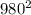
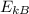 =
=