
Chemistry, 01.10.2019 19:00 kayyjayy3106
The work function of an element is the energy required to remove an electron from the surface of the solid element. the work function for lithium is 279.7 kj/mol (that is, it takes 279.7 kj of energy to remove one mole of electrons from one mole of li atoms on the surface of li metal). what is the maximum wavelength of light that can remove an electron from an atom on the surface of lithium metal?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:30, TheViperMlg23676
What causes most sediment to wash or fall into a river
Answers: 1

Chemistry, 22.06.2019 22:30, kristen17diaz
How many valence electrons are in atom of radon?
Answers: 1

Chemistry, 23.06.2019 03:00, winterblanco
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3
You know the right answer?
The work function of an element is the energy required to remove an electron from the surface of the...
Questions in other subjects:


History, 19.10.2019 00:00






Geography, 19.10.2019 00:00

English, 19.10.2019 00:00

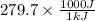
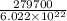
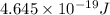
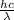
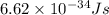
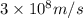
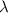 = wavelength
= wavelength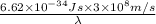
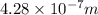
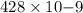 m
m


