
Chemistry, 01.10.2019 19:00 F00Dislife
Consider the equation: 2no(0) - n. using only the information given by the equation which of the following
changes would increase the molar concentration at equilibrium of the product n, o4(0)?
1)increase the temperature
2)decrease the pressure
3)decrease the temperature
4)decrease the concentration of no ala)
5)increase the pressure

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:30, americanbellabeauty
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
You know the right answer?
Consider the equation: 2no(0) - n. using only the information given by the equation which of the fo...
Questions in other subjects:


Mathematics, 29.04.2021 17:20





Social Studies, 29.04.2021 17:20


Physics, 29.04.2021 17:20

History, 29.04.2021 17:20

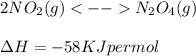

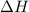 is not given
is not given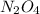 is not produced in greater amount.
is not produced in greater amount.


