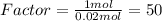Chemistry, 01.10.2019 18:20 poptropic9207
Epsom salts is a hydrated ionic compound with the following formula: mgso4⋅xh2o a sample of epsom salts with a mass of 4.93 g is heated to drive off the water of hydration. the mass of the sample after complete dehydration is 2.41 g. find the number of waters of hydration (x) in epsom salts

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:30, reaperqueen21
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 17:20, alexis3060
How do you know when a chemical reaction has occurred
Answers: 1
You know the right answer?
Epsom salts is a hydrated ionic compound with the following formula: mgso4⋅xh2o a sample of epsom s...
Questions in other subjects:


Mathematics, 10.11.2021 17:20


World Languages, 10.11.2021 17:20






English, 10.11.2021 17:20

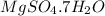
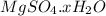 .After complete dehydration we have 2.41 g of
.After complete dehydration we have 2.41 g of 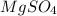 .
.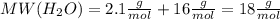
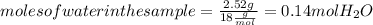
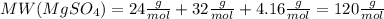
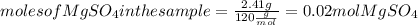
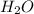 . Let´s find the multiplying factor to obtain the forula for 1 mol of
. Let´s find the multiplying factor to obtain the forula for 1 mol of