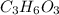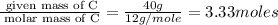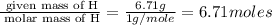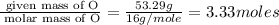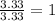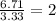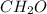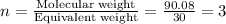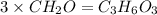Chemistry, 01.10.2019 17:30 MansellS5529
An elemental analysis is performed in an unknown compound. it is found to contain 40.0 % mass in carbon, 6.71% mass in hydrogen, and the remaining mass in oxygen. determine its empirical formula. the formula mass of the unknown is independently determined to be 90.08 g/mol, determine the unknown’s molecular formula

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:20, icantspeakengles
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 22.06.2019 23:00, aly02
Need asap question 1 minerals are organic compounds. true false question 2 what vitamin can be found in foods like oranges, grapefruits, and broccoli? a. vitamin a b. vitamin k c. vitamin c d. vitamin d question 3 what are minerals? a. chemical elements that are needed for body processes. b. organic compounds that the body needs in small amounts to function properly. c. small molecules used to build proteins. d. an organic compound that is insoluble in water and includes fats. question 4 how many types of vitamins does the human body need? a. 15 b. 11 c. 13 d. 17 question 5 vitamins are a good source of energy. true false
Answers: 1

Chemistry, 23.06.2019 07:30, lucas2020197
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 2

Chemistry, 23.06.2019 14:00, kcutler8603
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 14.0 mol cesium fluoride with 14.0 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1
You know the right answer?
An elemental analysis is performed in an unknown compound. it is found to contain 40.0 % mass in car...
Questions in other subjects:

Mathematics, 18.12.2019 15:31



Social Studies, 18.12.2019 15:31

Biology, 18.12.2019 15:31





History, 18.12.2019 15:31