
Chemistry, 01.10.2019 17:30 lasdivisionst6459
Within a single molecule of water, two hydrogen bonds are bonded to a single oxygen atom when there are multiple water molecules, the partial negative charge at one end of a water molecule attracted to the partial ositive charge of another water molecule forms

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, XxrazorxX11
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 05:40, yah2muchh
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 05:50, aylengarcia090
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 06:00, nikejose11
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3
You know the right answer?
Within a single molecule of water, two hydrogen bonds are bonded to a single oxygen atom when there...
Questions in other subjects:

Mathematics, 20.05.2021 01:00



History, 20.05.2021 01:00


Chemistry, 20.05.2021 01:00


History, 20.05.2021 01:00


Mathematics, 20.05.2021 01:00

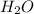 in which the two hydrogen atoms are connected to the oxygen atom via covalent bonds which means that the electrons are shared in the bond.
in which the two hydrogen atoms are connected to the oxygen atom via covalent bonds which means that the electrons are shared in the bond.

