
Chemistry, 01.10.2019 04:20 gadgetady5699
The gas arsine (ash3) decomposes as follows: 2ash3(g) < > 2as(s) + 3h2(g) in an experiment pure ash3(g) was placed in an empty, rigid, sealed flask at a pressure of 392.0 torr. after 48 h the pressure in the flask was observed to be constant at 488.0 torr. a. calculate the equilibrium pressure of h2(g). b. calculate kp for this reaction.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1


Chemistry, 22.06.2019 21:30, liamgreene90
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 22.06.2019 23:00, brianfranklin17
What is the correct lewis dot structure for arsenic?
Answers: 2
You know the right answer?
The gas arsine (ash3) decomposes as follows: 2ash3(g) < > 2as(s) + 3h2(g) in an experiment p...
Questions in other subjects:



Mathematics, 24.01.2022 23:50

Mathematics, 24.01.2022 23:50

English, 24.01.2022 23:50

History, 24.01.2022 23:50



Mathematics, 24.01.2022 23:50

Mathematics, 24.01.2022 23:50

 gas is, 288 torr
gas is, 288 torr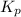 for this reaction is, 0.786 atm
for this reaction is, 0.786 atm


 at equilibrium = (392.0-2x) = [392.0-2(96)] = 200 torr
at equilibrium = (392.0-2x) = [392.0-2(96)] = 200 torr




