
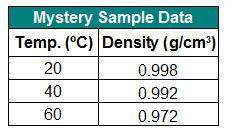
The table below shows the density of a sample of a mystery liquid you tested in the lab. can you infer the identity of the substance from these data?
a. yes, the substance must be water.
b. no, more data are needed.
c. no, the data must be wrong because density always decreases with an increase in temperature.
d. yes, but only if the data for 50ºc and 70ºc were also present.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, anthony4034
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1


Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1
You know the right answer?
The table below shows the density of a sample of a mystery liquid you tested in the lab. can you inf...
Questions in other subjects:


Mathematics, 02.07.2019 00:00


Mathematics, 02.07.2019 00:00



Spanish, 02.07.2019 00:00

Mathematics, 02.07.2019 00:00


History, 02.07.2019 00:00



