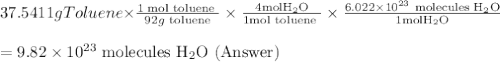One of the compounds used to increase the octane rating of gasoline is toluene (pictured). suppose 43.3 ml of toluene (d = 0.867 g/ml) is consumed when a sample of gasoline burns in air. how many grams of oxygen are needed for complete combustion of the toluene? (a) how many grams of oxygen are needed for complete combustion of the toluene? g (b) how many total moles of gaseous products form? mol (c) how many molecules of water vapor form?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, ethanmel21
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 08:30, lpssprinklezlps
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3


Chemistry, 22.06.2019 19:40, jholland03
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1
You know the right answer?
One of the compounds used to increase the octane rating of gasoline is toluene (pictured). suppose 4...
Questions in other subjects:



Mathematics, 01.12.2020 22:20

Mathematics, 01.12.2020 22:20


Mathematics, 01.12.2020 22:20

Biology, 01.12.2020 22:20

Mathematics, 01.12.2020 22:20


History, 01.12.2020 22:20

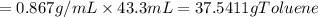

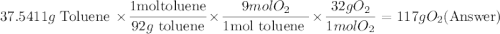
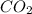 gas and 4 moles of
gas and 4 moles of 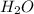 Vapour
Vapour
 molecules
molecules