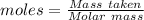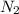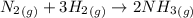Ammonia is produced by the reaction of nitrogen and hydrogen according to the equation n2(g) + 3h2(g) → 2nh3(g) calculate the mass of ammonia produced when 37.0 g of nitrogen react with 12.0 g of hydrogen. g nh3 which is the excess reactant and how much of it will be left over when the reaction is complete? hydrogen nitrogen

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, TheViperMlg23676
What causes most sediment to wash or fall into a river
Answers: 1

Chemistry, 22.06.2019 23:10, RealStephani
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(s o4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 22.06.2019 23:30, ninilizovtskt
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
Ammonia is produced by the reaction of nitrogen and hydrogen according to the equation n2(g) + 3h2(g...
Questions in other subjects:

Physics, 17.12.2020 18:10

Advanced Placement (AP), 17.12.2020 18:10

Mathematics, 17.12.2020 18:10


Mathematics, 17.12.2020 18:10

History, 17.12.2020 18:10




Mathematics, 17.12.2020 18:10

 .
.