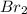Chemistry, 30.09.2019 21:10 brendacauani12345
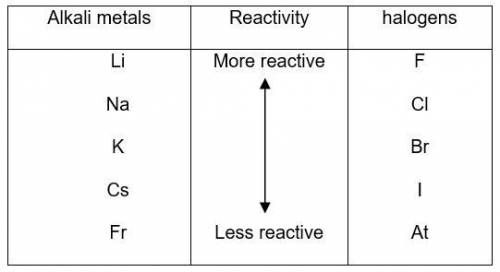
The elements in alkali metal and halogen groups of the periodic table are the most reactive since they only need to gain or lose one electron to become stable by filling their valence orbital. what happens to reactivity, moving down the column of a group?
a) reactivity stays the same because they are in the same group.
b) reactivity increases because the valence level is further from the nucleus of the atom.
c) reactivity decreases because the increased number of protons and electrons provides more stability.
d) reactivity stays the same because the number of electrons needed to fill the valence orbital remains the same.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, ulilliareinhart2
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 04:30, anthony4034
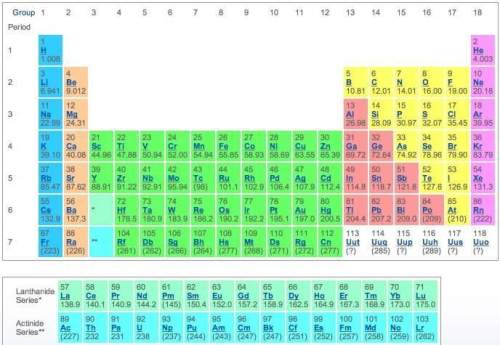
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1


Chemistry, 22.06.2019 09:50, Amandachavez94
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1
You know the right answer?
The elements in alkali metal and halogen groups of the periodic table are the most reactive since th...
Questions in other subjects:

History, 19.07.2019 14:20

Spanish, 19.07.2019 14:20

Computers and Technology, 19.07.2019 14:20

Mathematics, 19.07.2019 14:30




Mathematics, 19.07.2019 14:30

English, 19.07.2019 14:30

Mathematics, 19.07.2019 14:30

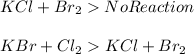
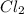 is more reactive than
is more reactive than