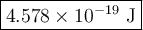Chemistry, 28.09.2019 23:10 zacksoccer8279
The blue line of the hydrogen emission spectrum has a wavelength of 433.9 nm. a hydrogen emission spectrum has a violet, a blue, a teal, and a red line. calculate the energy of one photon of this light.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, ayahabdulhaqq2
Nickel crystallizes in the face-centered cubic (fcc) lattice. the density of the metal is 8902 kg/m3. calculate the radius of a nickel atom.
Answers: 1

Chemistry, 21.06.2019 22:00, tgraveslaylay2743
Bose-einstein condensation occurs at what temperature?
Answers: 2

Chemistry, 22.06.2019 11:10, hannah2757
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1
You know the right answer?
The blue line of the hydrogen emission spectrum has a wavelength of 433.9 nm. a hydrogen emission sp...
Questions in other subjects:

Mathematics, 23.02.2021 19:10

Chemistry, 23.02.2021 19:10

History, 23.02.2021 19:10



Mathematics, 23.02.2021 19:10

Mathematics, 23.02.2021 19:10



English, 23.02.2021 19:10