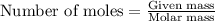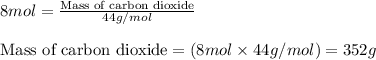Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, aleilyg2005
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 06:00, rebeccacruzz2017
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 12:30, ethanw8973
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 15:20, munziruddin204
Which description best characterizes the motion of particles in a solid?
Answers: 2
You know the right answer?
Enter your answer in the provided box. the balanced equation for the combustion of ethanol (ethyl al...
Questions in other subjects:

Biology, 19.09.2021 18:50




Mathematics, 19.09.2021 19:00



Health, 19.09.2021 19:00

Mathematics, 19.09.2021 19:00

Physics, 19.09.2021 19:00

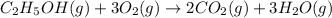
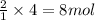 of carbon dioxide
of carbon dioxide