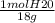Chemistry, 28.09.2019 03:10 marendt2014
Calculate the amount of heat in kj that is required to heat 25.0 g of ice from -25 °c to 105 °c in a closed vessel and sketch a heating curve for the process. the specific heat of ice is 2.11 j/(g. "c); 4.18 j/g. "c) for water, 2.00 j/g. "c. ahus for water is 6,01 kj/mol; ahp for water = 40.67 kj/mol.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, YoEsMyles3115
0.2348 grams of pbcl2 used to form 44.0 ml of solution.
Answers: 1

Chemistry, 22.06.2019 10:50, adam1299
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments, solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 18:30, losalobos46
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 22.06.2019 22:30, lanashanabJHsbd1099
Who discovered a pattern to the elements in 1869?
Answers: 1
You know the right answer?
Calculate the amount of heat in kj that is required to heat 25.0 g of ice from -25 °c to 105 °c in a...
Questions in other subjects:


History, 28.03.2021 07:30





Mathematics, 28.03.2021 07:30

Mathematics, 28.03.2021 07:40

English, 28.03.2021 07:40

History, 28.03.2021 07:40



 x
x