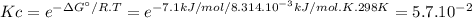Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:40, natannale
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1

Chemistry, 22.06.2019 05:00, happy121906
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 10:30, Brookwiggington8814
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 11:00, snowprincess99447
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1
You know the right answer?
Ag' for the isomerization reaction glucose-1-phosphate (gip) $ glucose-6-phosphate (g6p) is -7.1 kj/...
Questions in other subjects:

Mathematics, 05.05.2021 19:10

SAT, 05.05.2021 19:10


History, 05.05.2021 19:10

History, 05.05.2021 19:10



Chemistry, 05.05.2021 19:10

Mathematics, 05.05.2021 19:10

Mathematics, 05.05.2021 19:10

![Kc=\frac{[G1P]}{[G6P]}](/tpl/images/0269/8214/83bf5.png)