
Chemistry, 28.09.2019 02:20 IsabellaGracie
Two moles of ideal he gas are contained at a pressure of 1 atm and a temperature of 300 k. 34166 j of heat are transferred to the gas, as a result of which the gas expands and does 1216 j of work against its surroundings. the process is reversible. (note: c = 1.5r) calculate the final temperature of the gas

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:10, ChaseRussell24
Atank contains 240 liters of fluid in which 10 grams of salt is dissolved. brine containing 1 gram of salt per liter is then pumped into the tank at a rate of 6 l/min; the well-mixed solution is pumped out at the same rate. find the number a(t) of grams of salt in the tank at time t.
Answers: 3

Chemistry, 22.06.2019 08:40, jaueuxsn
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2


Chemistry, 22.06.2019 16:50, briansalazar17
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3
You know the right answer?
Two moles of ideal he gas are contained at a pressure of 1 atm and a temperature of 300 k. 34166 j o...
Questions in other subjects:


History, 11.03.2021 20:10



Mathematics, 11.03.2021 20:10


Arts, 11.03.2021 20:10

Mathematics, 11.03.2021 20:10


 =
= 
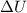 ) = Q + W
) = Q + W






