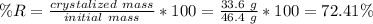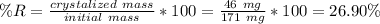Chemistry, 28.09.2019 02:20 kingofguns2826
the solubility of acetanilide is 12.8 g in 100 ml of ethanol at 0 ∘c, and 46.4 g in 100 ml of ethanol at 60 ∘c. what is the maximum percent recovery that can be achieved for the recrystallization of acetanilide from ethanol?
a student was given a sample of crude acetanilide to recrystallize. the initial mass of the the crude acetanilide was 171 mg. the mass after recrystallization was 125 mg.
calculate the percent recovery from recrystallization.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, veronica022
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 23:30, billybob8514
To find the work done, the force exerted and distance moved are multiplied. a couch is moved twice before you are happy with its placement. the same force was used to move the couch both times. if more work is done the first time it is moved, what do you know about the distance it was moved? a) when more work was done, the couch was moved the same distance. b) when more work was done, the couch was moved less. c) when more work was done, the couch was moved further. d) when more work was done, the couch wasn't moved at all.
Answers: 1

Chemistry, 23.06.2019 00:00, maronetham6253
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2
You know the right answer?
the solubility of acetanilide is 12.8 g in 100 ml of ethanol at 0 ∘c, and 46.4 g in 100 ml of ethano...
Questions in other subjects:



English, 03.12.2020 21:10



Mathematics, 03.12.2020 21:10

Mathematics, 03.12.2020 21:10