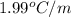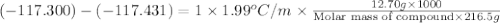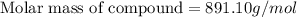Chemistry, 28.09.2019 01:30 emrecaga1992
The freezing point of ethanol, ch3ch2oh, is -117.300 °c at 1 atmosphere. kf(ethanol) = 1.99 °c/m
in a laboratory experiment, students synthesized a new compound and found that when 12.70 grams of the compound were dissolved in 216.5 grams of ethanol, the solution began to freeze at -117.431 °c. the compound was also found to be nonvolatile and a non-electrolyte.
what is the molecular weight they determined for this compound?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, heids17043
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 12:30, americanbellabeauty
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 16:00, corcoranrobert1959
How do dying stars contribute to the formation of planets
Answers: 1
You know the right answer?
The freezing point of ethanol, ch3ch2oh, is -117.300 °c at 1 atmosphere. kf(ethanol) = 1.99 °c/m
Questions in other subjects:

Computers and Technology, 30.05.2021 14:00


Mathematics, 30.05.2021 14:00


Computers and Technology, 30.05.2021 14:00

Geography, 30.05.2021 14:00

Chemistry, 30.05.2021 14:00

Physics, 30.05.2021 14:00



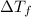 = change in freezing point
= change in freezing point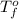 = temperature of pure ethanol =
= temperature of pure ethanol = 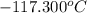
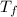 = temperature of solution =
= temperature of solution = 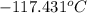
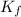 = freezing point constant of ethanol =
= freezing point constant of ethanol =