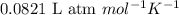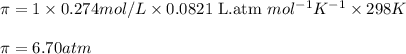Chemistry, 28.09.2019 01:30 taylorclmna
The nonvolatile, nonelectrolyte estrogen (estradiol), c18h24o2 (272.40 g/mol), is soluble in chloroform chcl3.
calculate the osmotic pressure generated when 13.5 grams of estrogen are dissolved in 181 ml of a chloroform solution at 298 k.
the molarity of the solution is ? m.
the osmotic pressure of the solution is ? atmospheres.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, mariamakonteh31
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1


Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
The nonvolatile, nonelectrolyte estrogen (estradiol), c18h24o2 (272.40 g/mol), is soluble in chlorof...
Questions in other subjects:

Physics, 20.07.2019 20:00

Physics, 20.07.2019 20:00

Mathematics, 20.07.2019 20:00


Physics, 20.07.2019 20:00


Mathematics, 20.07.2019 20:00


Mathematics, 20.07.2019 20:00

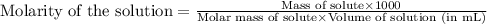
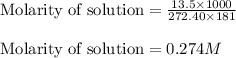
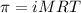
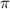 = osmotic pressure of the solution = ?
= osmotic pressure of the solution = ?