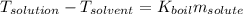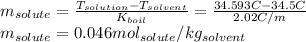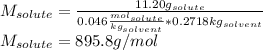The boiling point of diethyl ether, ch3ch2och2ch3, is 34.500 °c at 1 atmosphere. kb(diethyl ether) = 2.02 °c/m
in a laboratory experiment, students synthesized a new compound and found that when 11.20 grams of the compound were dissolved in 271.8 grams ofdiethyl ether, the solution began to boil at 34.593 °c. the compound was also found to be nonvolatile and a non-electrolyte.
what is the molecular weight they determined for this compound ?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:10, mistiehaas
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 09:30, andrejr0330jr
What is the molar mass of potassium nitrate, kno3
Answers: 1

Chemistry, 22.06.2019 19:00, miguel454545
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 21:00, andrethisman88
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
You know the right answer?
The boiling point of diethyl ether, ch3ch2och2ch3, is 34.500 °c at 1 atmosphere. kb(diethyl ether) =...
Questions in other subjects:









Mathematics, 12.10.2020 01:01