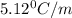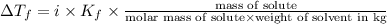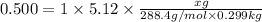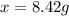How many grams of testosterone, c19h28o2 (288.4 g/mol), must be dissolved in 299.0 grams of benzene to reduce the freezing point by 0.500°c ? refer to the table for the necessary boiling or freezing point constant.
solvent
formula
kb (°c/m)
kf (°c/m)
water
h2o
0.512
1.86
ethanol
ch3ch2oh
1.22
1.99
chloroform
chcl3
3.67
benzene
c6h6
2.53
5.12
diethyl ether
ch3ch2och2ch3
2.02
? g testosterone.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:30, allofthosefruit
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 22.06.2019 21:00, agarcia24101993
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 22.06.2019 22:30, teagan56
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
You know the right answer?
How many grams of testosterone, c19h28o2 (288.4 g/mol), must be dissolved in 299.0 grams of benzene...
Questions in other subjects:

SAT, 02.02.2022 14:00



Chemistry, 02.02.2022 14:00

SAT, 02.02.2022 14:00




Computers and Technology, 02.02.2022 14:00

Mathematics, 02.02.2022 14:00

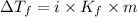
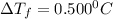 = Depression in freezing point
= Depression in freezing point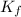 = freezing point constant of benzene=
= freezing point constant of benzene=