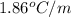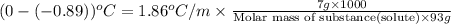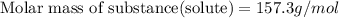Chemistry, 28.09.2019 01:20 xxxamslashxxx9
Calculate the molecular weight of a substance. in which the solution of this substance in the water has a concentration of 7 percent by weight, has a freezing point
equal to -0.89 ° c, set kf value of water = 1.86 ° c / m

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:00, 5041
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1


You know the right answer?
Calculate the molecular weight of a substance. in which the solution of this substance in the water...
Questions in other subjects:


English, 08.10.2020 07:01



Spanish, 08.10.2020 07:01



Mathematics, 08.10.2020 07:01


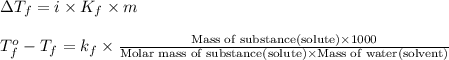
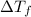 = change in freezing point
= change in freezing point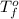 = temperature of pure water =
= temperature of pure water = 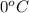
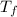 = temperature of solution =
= temperature of solution = 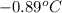
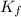 = freezing point constant of water =
= freezing point constant of water =