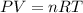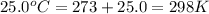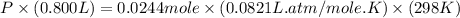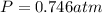Solid potassium chlorate decomposes into solid potassium chloride and oxygen gas. if 2.00 g potassium chlorate decomposes in a chamber with a fixed volume of 0.800 l, and temperature of 25.0◦c. what is the final chamber pressure? assume all the potassium chlorate decomposes. express your answer in atmospheres.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:20, paulawells11
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 21:20, skyemichellec
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

Chemistry, 22.06.2019 22:30, lizzzzi7908
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1
You know the right answer?
Solid potassium chlorate decomposes into solid potassium chloride and oxygen gas. if 2.00 g potassiu...
Questions in other subjects:






Computers and Technology, 23.01.2020 00:31

Social Studies, 23.01.2020 00:31

Social Studies, 23.01.2020 00:31


English, 23.01.2020 00:31

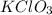 .
.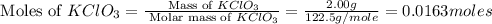
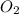 .
.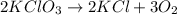
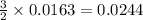 moles of
moles of