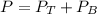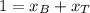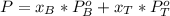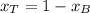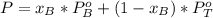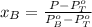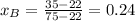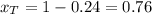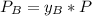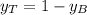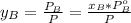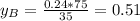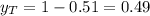Chemistry, 27.09.2019 04:10 chamarabrown9260
A) whatis the composition in mole fractions of a solution of benzene and toluene that has a vapor pressure of 35 torr at 20 °c? assume the mixture formsan ideal solution. the vapor pressure of benzene (c6h6) is 75 torr and the vapor pressure of toluene (c7h8)is 22 torr at 20 °c. b) what is the composition in mole fractions of the vapor above the solution in part a? how does this problem relate to the process of fractional distillation?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 00:30, lareynademividp0a99r
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1

Chemistry, 23.06.2019 09:20, weridness80
Which of the following occurs along coasts during the day?
Answers: 3

Chemistry, 23.06.2019 19:30, micahsocool23
The molar mass of cu is 63.55 g/mol. the number of grams of cu produced in this reaction is .
Answers: 1
You know the right answer?
A) whatis the composition in mole fractions of a solution of benzene and toluene that has a vapor pr...
Questions in other subjects:



Computers and Technology, 19.02.2021 19:30

Mathematics, 19.02.2021 19:30


Chemistry, 19.02.2021 19:30




Arts, 19.02.2021 19:30

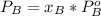
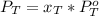
 is partial pressure for benzene in the liquid
is partial pressure for benzene in the liquid 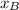 is benzene molar fraction in the liquid
is benzene molar fraction in the liquid 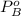 vapor pressure for pure benzene.
vapor pressure for pure benzene.